Ke wehe nei i ka mea pohihihi: Super Theoretical Capacity in Lithium-Ion Batteries
By hoppt
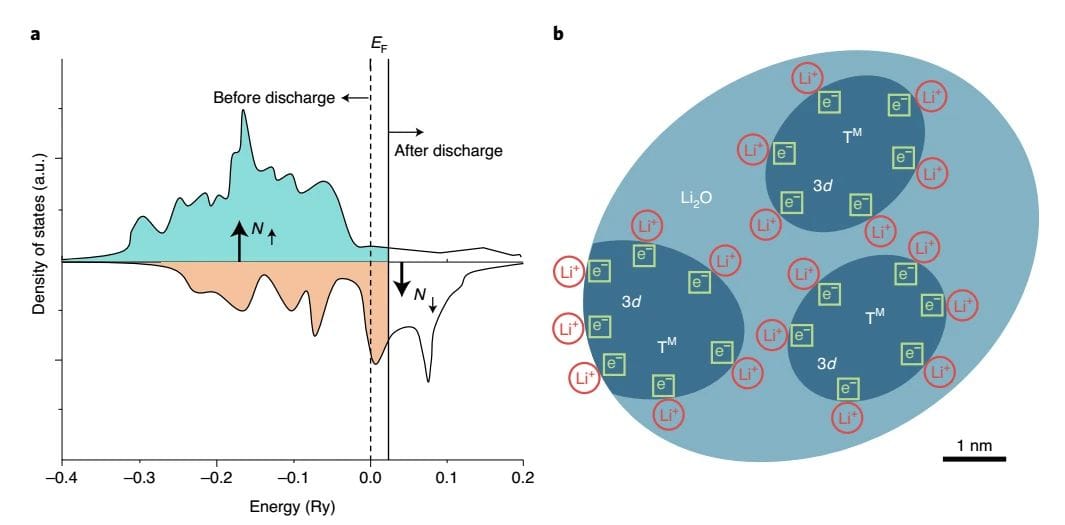
No ke aha i loaʻa ai ka pākaukau lithium super theoretical capacity phenomenon
I loko o nā pākahi lithium-ion (LIBs), hōʻike ka nui o nā electrodes hoʻololi metala oxide-based e hōʻike ana i ka nui o ka mālama ʻana ma mua o ko lākou kumu waiwai. ʻOiai ua hōʻike nui ʻia kēia ʻano, ʻo ke kumu physicochemical mechanical i loko o kēia mau mea e noho paʻakikī a hoʻopaʻapaʻa.
Ka moʻolelo o nā hualoaʻa
I kēia mau lā, ua paʻi pū ʻo Professor Miao Guoxing mai ke Kulanui o Waterloo, Kanada, ʻo Professor Yu Guihua mai ke Kulanui o Texas ma Austin, a me Li Hongsen lāua ʻo Li Qiang mai ke Kulanui ʻo Qingdao i kahi pepa noiʻi no Nature Materials ma lalo o ke poʻo inoa o "Extra storage capacity in i hōʻike ʻia e ka magnetometry in situ. Ma kēia hana, ua hoʻohana nā mea kākau i ka nānā magnetic in situ e hōʻike i ka hiki ʻana o ka capacitance ili ikaika ma nā nanoparticles metala a hiki ke mālama ʻia ka nui o nā electrons spin-polarized i loko o nā nanoparticles metala i hoʻemi ʻia, i kūlike me ka mīkini hoʻoili spatial. Eia hou, hiki ke hoʻonui ʻia ka mīkini hoʻoili spatial i hōʻike ʻia i nā pūhui metala hoʻololi ʻē aʻe, e hāʻawi ana i alakaʻi kumu no ka hoʻokumu ʻana i nā ʻōnaehana mālama ikehu holomua.
Nā manaʻo kiʻekiʻe noiʻi
(1) Ua aʻo ʻia kahi Fe maʻamau ma o ka hoʻohana ʻana i ka ʻenehana nānā magnetic in-situ3O4/ Evolution o ka hale uila i loko o ka pā Li;
(2) hōʻike i ka Fe3O4In ka / Li ʻōnaehana, ʻo ka mana o ka ʻili ke kumu nui o ka mana keu;
(3) Hiki ke hoʻonui ʻia ka mīkini capacitance surface o nā nanoparticles metala i kahi ākea o nā pūhui metala hoʻololi.
Kikokikona a me kikokiko alakaʻi
- ʻO ka hiʻohiʻona hoʻolālā a me nā waiwai electrochemical
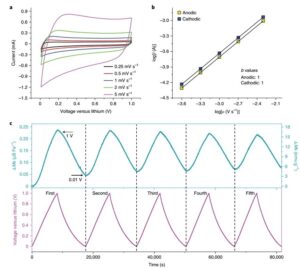
Hoʻohui ʻia ka Monodisperse hollow Fe e nā ʻano hydrothermal maʻamau3O4Nanospheres, a laila hana ʻia ma 100 mAg−1Charge a me ka hoʻokuʻu ʻana i ka nui o kēia manawa (Figure 1a), ʻo ka mana hoʻokuʻu mua ʻo 1718 mAh g−1, 1370 mAhg i ka lua a me ke kolu o ka manawa. 1 A me 1,364 mAhg−1, ʻoi aku ma mua o 926 mAhg−1Ka manaʻo o nā manaʻolana. Hōʻike nā kiʻi BF-STEM o ka huahana i hoʻopau piha ʻia (Figure 1b-c) ma hope o ka emi ʻana o ka lithium, Fe3O4The nanospheres ua hoʻololi ʻia i nā nanoparticles Fe liʻiliʻi e ana ma kahi o 1 - 3 nm, i hoʻopuehu ʻia i ke kikowaena Li2O.
No ka hōʻike ʻana i ka hoʻololi ʻana o ka magnetism i ka wā o ka pōʻaiapili electrochemical, ua loaʻa kahi pihi magnetization ma hope o ka hoʻokuʻu piha ʻana i ka 0.01 V (Figure 1d), e hōʻike ana i ke ʻano superparamagnetic ma muli o ka hoʻokumu ʻana o nā nanoparticles.
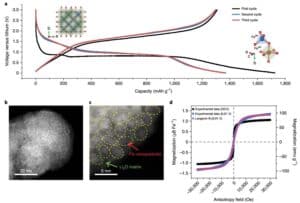
Kiʻi 1 (a) ma 100 mAg−1Fe o ke kaʻa kaʻa ma ka density o kēia manawa3O4/ Ka piʻi hoʻoiho mau a me ka hoʻokuʻu ʻana o Li; (b) lithium piha Fe3O4Ke kiʻi BF-STEM o ka electrode; (c) ka loaʻa ʻana o Li i nā kiʻi aggregate2High-resolution BF-STEM o nā O a me Fe; (d) Fe3O4Nā pihi hysteresis o ka electrode ma mua (ʻeleʻele) a ma hope (uliuli), a me ka pihi hoʻopili ʻo Langevin o ka hope (poni).
- ʻIke manawa maoli o ka hoʻomohala ʻana a me ka hoʻomohala magnetic
I mea e hoʻohui ai i ka electrochemistry me Fe3O4Of structural and magnetic changes i hoʻopili ʻia i ka Fe3O4The Electrodes were subjected in situ X-ray diffraction (XRD) and in situ magnetic monitoring. Fe i loko o ke kaʻina o XRD diffraction patterns i ka wā o ka hoʻokuʻu mua ʻana mai ka volta kaapuni hāmama (OCV) a i ka 1.2V3O4 ʻAʻole i loli nui nā piko o ka diffraction i ka ikaika a i ʻole ke kūlana (Figure 2a), e hōʻike ana ua ʻike ka Fe3O4Only i ke kaʻina intercalation Li. Ke hoʻoili ʻia i ka 3V, ʻo ka Fe3O4The anti-spinel structure e paʻa mau, e hōʻike ana he hiki ke hoʻohuli ʻia ke kaʻina hana ma kēia puka aniani. Ua hana ʻia ka mākaʻikaʻi magnetic in-situ i hui pū ʻia me nā hoʻāʻo hoʻokuʻu hoʻoiho mau i kēia manawa e noiʻi i ka ulu ʻana o ka magnetization i ka manawa maoli (Figure 2b).
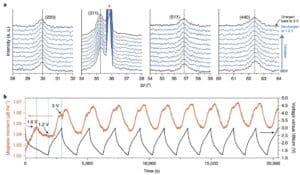
Kiʻi 2 ʻO ke ʻano o ka XRD in-situ a me ka nānā ʻana i ka magnetic. (A) in situ XRD; (b) Fe3O4Electrochemical charge-discharge curve ma lalo o 3 T i hoʻohana ʻia i ke kahua mākēneki a me ka hoʻihoʻi ʻia ʻana i loko o ka pane magnetic.
No ka loaʻa ʻana o ka ʻike maʻamau o kēia kaʻina hoʻololi e pili ana i nā loli magnetization, hōʻiliʻili ʻia ka pane magnetic i ka manawa maoli a me ka hoʻololi ʻana o ka pae e pili ana i nā hopena electrochemically driven (Figure 3). Akaka loa i ka wā o ka hoʻokuʻu mua ʻana, ʻokoʻa ka Fe3O4The magnetization pane o nā electrodes mai nā pōʻai ʻē aʻe ma muli o Fe i ka wā o ka lithization3O4 mua ma muli o ka hoʻololi ʻana o ka pae hiki ʻole. I ka hāʻule ʻana o ka hiki i 0.78V, ua hoʻohuli ʻia ka Fe3O4The antispinel phase i loaʻa i ka Li2The class FeO halite structure o O, Fe3O4 ʻAʻole hiki ke hoʻihoʻi ʻia ka pae ma hope o ke kau ʻana. E like me, hāʻule koke ka magnetization i 0.482 μ b Fe−1. Ke holo nei ka lithialization, ʻaʻohe mea hou i hoʻokumu ʻia, a ua hoʻomaka ka ikaika o ka (200) a me (220) papa FeO diffraction peaks e nāwaliwali. like Fe3O4 ʻAʻohe mea koʻikoʻi XRD peak i paʻa i ka wā e liialized loa ai ka electrode (Figure 3a). E hoʻomaopopo i ka wā e hoʻokuʻu ai ka electrode Fe3O4 mai 0.78V a i 0.45V, ka magnetization (mai 0.482 μ b Fe−1Hoʻonui i 1.266 μ bFe−1), Ua pili kēia i ka hoʻololi ʻana mai FeO a i Fe. A laila, i ka pau ʻana o ka hoʻokuʻu ʻana, ua emi mālie ka magnetization i 1.132 μ B Fe−1. Ke hōʻike nei kēia ʻike e hiki ke komo pū nā metala Fe0Nanoparticles i hoʻemi ʻia i ka hopena mālama lithium, no laila e hōʻemi ana i ka magnetization o nā electrodes.
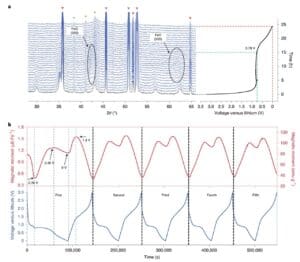
Kiʻi 3 Nā ʻike ma kahi o ka neʻe ʻana o ka māhele a me ka pane ʻana. (b) Fe3O4In situ ke ana ikaika o nā pōʻaiapili electrochemical o / Li ma kahi mākia i hoʻohana ʻia o 3 T.
- Fe0/Li2Surface capacitance o ka ʻōnaehana O
Fe3O4Ke hoʻololi ʻia ka hoʻololi ʻana o nā electrodes ma nā volta haʻahaʻa, kahi e hana ʻia ai kahi mana electrochemical hou, e hōʻike ana i ka loaʻa ʻana o nā mea lawe ukana i ʻike ʻole ʻia i loko o ke kelepona. No ka ʻimi ʻana i ka mīkini hoʻopaʻa lithium hiki, ua aʻo ʻia ʻo Fe ma o XPS, STEM a me ka spectrum3O4Electrodes o ka magnetization peaks ma 0.01V, 0.45V a me 1.4V e hoʻoholo ai i ke kumu o ka hoʻololi magnetic. Hōʻike nā hualoaʻa he kumu koʻikoʻi ka manawa magnetic e pili ana i ka hoʻololi magnetic, no ka mea, ʻaʻole i hoʻopili ʻia ka Fe0/Li2The Ms o ka ʻōnaehana O e ka anisotropy magnetic a me ka hoʻohui ʻana i waena.
No ka hoʻomaopopo hou aku i ka Fe3O4The kinetic properties of the electrodes at haʻahaʻa voltammetry, cyclic voltammetry ma nā ʻano scan like ʻole. E like me ka mea i hōʻike ʻia ma ke Kiʻi 4a, ʻike ʻia ka ʻāpana voltammogram cyclic rectangular i loko o ka pae uila ma waena o 0.01V a me 1V (Figure 4a). Hōʻike ka Figure 4b ua loaʻa ka pane capacitive Fe3O4A ma ka electrode. Me ka hoʻohuli nui ʻana i ka pane magnetic o ke kaʻina hana a me ka hoʻokuʻu ʻana i kēia manawa (Figure 4c), ua emi ka magnetization o ka electrode mai 1V a i 0.01V i ka wā o ka hoʻokuʻu ʻana, a hoʻonui hou i ka wā o ke kaʻina hana, e hōʻike ana i ka Fe0Of ka capacitor-like. Hiki ke hoʻohuli ʻia ka hopena o ka ʻili.
Helu 4 mau waiwai electrochemical a me in situ magnetic characterization ma 0.011 V. (A) Ka cyclic voltammetric curve. (B) ua hoʻoholo ʻia ka waiwai b me ka hoʻohana ʻana i ka pilina ma waena o ke au kiʻekiʻe a me ka helu scan; (c) ka hoʻololi hou ʻana o ka magnetization e pili ana i ka pihi hoʻokuʻu-hoʻokuʻu ma lalo o kahi kahua magnetic 5 T i hoʻohana ʻia.
Fe3O4 ʻO nā hiʻohiʻona electrochemical, structural a me magnetic o nā electrodes i hōʻike ʻia e hoʻoholo ʻia ka mana pākaukau hou e Fe0The spin-polarized surface capacitance o nā nanoparticles ma muli o nā hoʻololi magnetic e pili pū ana. ʻO ka capacitance spin-polarized ka hopena o ka hōʻiliʻili ʻana o ka spin-polarized charge ma ka interface a hiki ke hōʻike i kahi pane magnetic i ka wā o ka hoʻopiʻi a me ka hoʻokuʻu. ka nui o ka ilikai a me ka nui a ʻike i kahi kiʻekiʻe kiʻekiʻe o nā mokuʻāina ma ka pae Fermi ma muli o nā orbital d localized. Wahi a Maier ke kumu hoʻohālike o ka mālama ʻana i ka spatial charge, manaʻo nā mea kākau e hiki ke mālama ʻia ka nui o nā electrons i loko o nā ʻāpana spin-splitting o nā nanoparticles Fe metala, hiki ke loaʻa ma Fe / Li3Creating spin-polarized surface capacitors i ka O nanocomposites Helu 4).
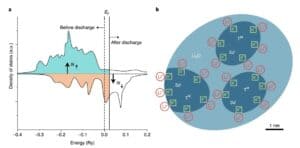
graph 5Fe/Li2A Hōʻike manaʻo o ka capacitance ili o nā electrons spin-polarized ma ka O-interface. ka polarization milo nui o ka hao; (b) ka hoʻokumu ʻana o ka ʻāpana hoʻopaʻa lewa i ke kumu hoʻohālike capacitor o ka lithium overstored.
Hōʻuluʻulu manaʻo a me Outlook
Ua noiʻi ʻia ʻo TM / Li e ka mākaʻikaʻi magnetic in-situ kiʻekiʻe2 ʻO ka hoʻololi ʻana o ka ʻōnaehana uila kūloko o ka nanocomposite O e hōʻike i ke kumu o ka hiki ke mālama hou no kēia pākaukau lithium-ion. Hōʻike nā hualoaʻa, i loko o ka Fe3O4/Li model cell system, electrochemically reduced Fe nanoparticles hiki ke mālama i ka nui o nā electrons spin-polarized, ka hopena ma muli o ka nui o ke kelepona a me ka hoʻololi nui ʻana i ka magnetism interfacial. Ua hōʻoia hou nā hoʻokolohua CoO, NiO, a me FeF2A me Fe2 ʻO ka loaʻa ʻana o ia capacitance i ka N electrode material e hōʻike ana i ke ʻano o ka spin-polarized surface capacitance o nā nanoparticles metala i nā pā lithium ion a kau i ke kumu no ka hoʻohana ʻana i kēia mīkini mālama mālama spatial i nā hoʻololi ʻē aʻe. nā mea electrode ma muli o ka metala.
loulou palapala
ʻO ka hiki ke hoʻopaʻa ʻia i nā pākahi lithium-ion metala metala i hōʻike ʻia e in situ magnetometry (Nature Materials, 2020, DOI: 10.1038/s41563-020-0756-y)
Ka mana o ka lithium electrode wafer design formula a me electrode wafer defects i ka hana
- ʻatikala kumu kiʻi ʻoniʻoni pole
ʻO ka lithium battery electrode he uhi i haku ʻia me nā ʻāpana, i hoʻopili pono ʻia i ka wai metala. Hiki ke noʻonoʻo ʻia ka uhi ʻana o ka pā uila Lithium ion ma ke ʻano he mea hoʻohui, ʻo ka nui o nā ʻāpana ʻekolu:
(1) Nā ʻāpana mea hana ikaika;
(2) ka mahele constituent o ka mea hana conductive a me ka mea hana (carbon adhesive phase);
(3) Pore, hoʻopiha me ka electrolyte.
Hōʻike ʻia ka pilina leo o kēlā me kēia māhele penei:
Porosity + haʻihaʻi leo mea ola + hakina puʻupuʻu kalapona hoʻopili =1
He mea koʻikoʻi ka hoʻolālā ʻana o ka hoʻolālā electrode lithium battery, a i kēia manawa ua hōʻike pōkole ʻia ka ʻike kumu o ka hoʻolālā electrode lithium battery.
(1) Theoretical capacity of the electrode material ʻO ka mana theoretical o ka mea electrode, ʻo ia hoʻi, ka mana i hāʻawi ʻia e nā ion lithium āpau i loko o ka mea i komo i ka hopena electrochemical, helu ʻia kona waiwai e ka hoʻohālikelike aʻe:
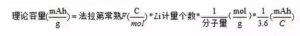
No ka laʻana, ʻo ka LiFePO4The molar mass he 157.756 g/mol, a ʻo kona mana manaʻo:
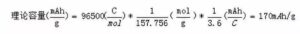
ʻO kēia waiwai helu wale nō ka mana gram theoretical. I mea e hōʻoiaʻiʻo ai i ka hoʻololi hou ʻana o ka mea, ʻoi aku ka liʻiliʻi o ka lithium ion removal coefficient ma mua o 1, a ʻo ka mana gram maoli o ka mea:
ʻO ka mana gram maoli o nā mea = ka mana o ka lithium ion unplugging coefficient
(2) Hiki ke helu ʻia ka mana hoʻolālā pākaukau a me ka ʻaoʻao hoʻokahi ʻaoʻao hiki ke helu ʻia ka hiki ke hoʻolālā ʻia ma ke ʻano penei: hiki ke hoʻolālā ʻia i ka pākaukau = ka uhi ʻana i ka ʻili o ka ʻili.
I waena o lākou, ʻo ka nui o ka ʻili o ka uhi ʻana he ʻano hoʻolālā koʻikoʻi. Ke hoʻololi ʻole ka pāpaʻi compaction, ʻo ka hoʻonui ʻana o ka ʻili o ka uhi uhi ʻo ia ka mea e piʻi ai ka mānoanoa o ka pole, piʻi ka mamao o ka lawe ʻana i ka electron, a piʻi ke kūʻē electron, akā ua kaupalena ʻia ke degere hoʻonui. I ka pepa electrode mānoanoa, ʻo ka piʻi ʻana o ka impedance neʻe o nā ion lithium i ka electrolyte ke kumu nui e pili ana i nā ʻano ratio. Ke noʻonoʻo nei i ka porosity a me ka pore twists, ʻoi aku ka nui o ka neʻe ʻana o nā ion i loko o ka pore ma mua o ka mānoanoa o ka pepa pole.
(3) Ua wehewehe ʻia ka lakio o ka lākiō ʻino-maikaʻi maikaʻi ʻole N / P hiki ʻole i ka hiki ke kūpono:

ʻOi aku ka nui o N / P ma mua o 1.0, maʻamau 1.04 ~ 1.20, ʻo ia ka mea nui i ka hoʻolālā palekana, e pale i ka ʻaoʻao maikaʻi ʻole lithium ion mai ka ua me ka ʻole o ka ʻae ʻana, e hoʻolālā e noʻonoʻo i ke kaʻina hana, e like me ka pale ʻana. Eia nō naʻe, inā nui ka nui o ka N / P, e nalowale ka pākaukau i ka hiki ʻole ke hoʻihoʻi ʻia, e hopena i ka haʻahaʻa haʻahaʻa a me ka haʻahaʻa haʻahaʻa.
No ka lithium titanate anode, ua lawe ʻia ka hoʻolālā hoʻolālā electrode maikaʻi, a ua hoʻoholo ʻia ka mana pākaukau e ka hiki o ka lithium titanate anode. ʻO ka hoʻolālā hoʻolālā maikaʻi e kūpono i ka hoʻomaikaʻi ʻana i ka hana wela kiʻekiʻe o ka pākaukau: ʻo ke kinoea wela kiʻekiʻe e hele mai mai ka electrode maikaʻi ʻole. Ma ka hoʻolālā hoʻolālā maikaʻi, haʻahaʻa ka mana maikaʻi ʻole, a ʻoi aku ka maʻalahi o ka hana ʻana i ke kiʻi SEI ma ka ʻili o ka lithium titanate.

(4) Compaction density and porosity of the coating I ke kaʻina hana, ua helu ʻia ka hoʻopili paʻa paʻa ʻana o ka electrode pākaukau e ke ʻano ma lalo nei. E noʻonoʻo ana i ka wā e ʻōwili ʻia ai ka pepa pou, ua hoʻolōʻihi ʻia ka pahu metala, ua helu ʻia ka nui o ka ʻili o ka uhi ma hope o ka ʻōwili ʻia e kēia ʻano.
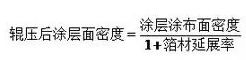
E like me ka mea i ʻōlelo ʻia ma mua, ʻo ka uhi ʻana o ka pae mea ola, carbon adhesive phase a me pore, a hiki ke helu ʻia ka porosity e ka hoʻohālikelike aʻe.
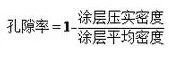
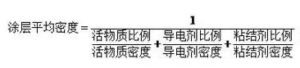
I waena o lākou, ka awelika density o ka uhi mea: lithium pākahiko electrode mea he 'ano o ka pauka particles o ka uhi, no ka mea, o ka pauka ili ili oolea, irregular shape, i ka wa accumulation, particles ma waena o particles a me na particles, a me kekahi mau particles iho i mau māwae a me pores, no laila, ka nui o ka pauka me ka nui o ka pauda, nā pores ma waena o nā ʻāpana pauda a me nā ʻāpana, no laila, ke ʻano like ʻole o ka nui o ka electrode coating density a me ka hōʻike porosity. ʻO ka mānoanoa o nā ʻāpana pauda e pili ana i ka nuipaʻa o ka pauda ma kēlā me kēia leo. E like me ka nui o ka pauka, ua māhele ʻia i ʻekolu mau ʻano: ʻoiaʻiʻo maoli, ʻāpana ʻāpana a me ka nui o ka hōʻiliʻili. Ua wehewehe ʻia nā ʻano like ʻole penei:
- ʻO ka mānoanoa ʻoiaʻiʻo e pili ana i ka mānoanoa i loaʻa ma ka puʻunaue ʻana i ka nui o ka pauda me ka leo (leo maoli) me ka ʻole o nā āpau o loko a me waho o nā ʻāpana. ʻO ia hoʻi, ka nui o ka mea i loaʻa iā ia iho ma hope o ka wehe ʻana i ka leo o nā voids a pau.
- ʻO ka mānoanoa o nā ʻāpana e pili ana i ka mānoanoa o nā ʻāpana i loaʻa ma ka puʻunaue ʻana i ka pauka pauda i puʻunaue ʻia e ka nui o nā ʻāpana me ka puka hāmama a me ka puka pani. ʻO ia hoʻi, ka ʻokoʻa ma waena o nā ʻāpana, ʻaʻole naʻe nā pores maikaʻi i loko o nā ʻāpana, ʻo ka mānoanoa o nā ʻāpana ponoʻī.
- ʻO ka puʻupuʻu hōʻiliʻili, ʻo ia hoʻi, ka uhi ʻana, e pili ana i ka nui i loaʻa e ka pauka pauka i puʻunaue ʻia e ka nui o ka uhi i hana ʻia e ka pauka. ʻO ka leo i hoʻohana ʻia e pili ana i nā pores o nā ʻāpana iā lākou iho a me nā puka i waena o nā ʻāpana.
No ka pauka hoʻokahi, ʻoiaʻiʻo ka nui> ʻāpana ʻāpana> hoʻopaʻa paʻa. ʻO ka porosity o ka pauka ka lākiō o nā pores i loko o ka uhi ʻana o ka pauka, ʻo ia hoʻi, ka ratio o ka nui o ka ʻole ma waena o nā ʻāpana pauka a me nā pores o nā ʻāpana i ka nui o ka nui o ka uhi, i hōʻike pinepine ʻia. ma ke ʻano he pākēneka. ʻO ka porosity o ka pauka kahi waiwai piha e pili ana i ka morphology particle, state surface, particle size and particle size distribution. Hoʻopili pololei kona porosity i ka infiltration o ka electrolyte a me ka lithium ion transmission. Ma keʻano laulā, ʻoi aku ka nui o ka porosity, ʻoi aku ka maʻalahi o ka infiltration electrolyte, a ʻoi aku ka wikiwiki o ka lawe ʻana i ka lithium ion. No laila, i ka hoʻolālā ʻana o ka pā lithium, i kekahi manawa e hoʻoholo ai i ka porosity, hoʻohana maʻamau i ke ʻano o ke kaomi mercury, ke ʻano adsorption gas, etc. Hiki ke loaʻa ma ka hoʻohana ʻana i ka helu density. Hiki i ka porosity ke loaʻa nā hopena like ʻole i ka hoʻohana ʻana i nā density like ʻole no ka helu ʻana. Ke helu ʻia ka mānoanoa o ka porosity o ka mea ola, ka mea hoʻokele a me ka mea hoʻopaʻa paʻa e ka paʻa maoli, ʻo ka porosity i helu ʻia e pili ana i ka āpau ma waena o nā ʻāpana a me ka ʻāpana i loko o nā ʻāpana. Ke helu ʻia ka porosity o ka mea ola, conductive agent a me ka mea hoʻopaʻa paʻa e ka pākuʻi ʻāpana, ʻo ka porosity i helu ʻia e pili ana i ke āpau ma waena o nā ʻāpana, ʻaʻole naʻe ka āpau i loko o nā ʻāpana. No laila, ʻo ka nui o ka pore o ka lithium battery electrode sheet he multi-scale, ma ke ʻano maʻamau, ʻo ke āpau ma waena o nā ʻāpana i ka nui o ka micron scale, ʻoiai ʻo ke āpau i loko o nā ʻāpana i loko o ka nanometer a i ka unahi sub-submicron. I loko o nā electrodes porous, hiki ke hōʻike ʻia ka pilina o nā waiwai lawe e like me ka diffusivity kūpono a me ka conductivity e ka hoohalike aʻe:
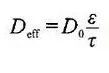
Ma kahi e hōʻike ai ʻo D0 i ka laiki diffusion intrinsic (conduction) o ka mea ponoʻī, ʻo ε ka hakina leo o ka māhele pili, a ʻo τ ka curvature kaapuni o ka pae pili. I loko o ka macroscopic homogeneous kŘkohu, hoʻohana maʻamau ka pilina Bruggeman, e lawe ana i ka coefficient ɑ =1.5 e hoʻohālikelike i ka positivity kūpono o nā electrodes porous.
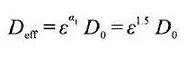
Hoʻopihaʻia ka electrolyte i nā pores o nā electrodes porous, kahi e alakaʻiʻia ai nā lithium ion ma o ka electrolyte, aʻo nāʻano conduction o nā lithium ion e pili pono i ka porosity. ʻO ka nui o ka porosity, ʻoi aku ka kiʻekiʻe o ka hapa leo o ka pae electrolyte, a ʻoi aku ka nui o ka conductivity pono o nā ion lithium. Ma ka pepa electrode maikaʻi, hoʻouna ʻia nā electrons ma o ka pae kalapona adhesive, ʻo ka hapa leo o ka māhele kalapona adhesive a me ka hele ʻana o ka manawa adhesive carbon e hoʻoholo pololei i ka conductivity kūpono o nā electrons.
ʻO ka porosity a me ka hapa leo o ka carbon adhesive phase he kue, a ʻo ka porosity nui inevitably alakaʻi i ka hapa leo o ka carbon adhesive phase, no laila, kūʻē pū nā waiwai conduction pono o nā ion lithium a me nā electrons, e like me ka hōʻike ʻana ma ke Kiʻi 2 I ka emi ʻana o ka porosity, e emi ana ka conductivity pono lithium ion i ka wā e hoʻonui ai ka conductivity pono electron. Pehea e kaulike ai i nā mea ʻelua he mea koʻikoʻi hoʻi i ka hoʻolālā electrode.
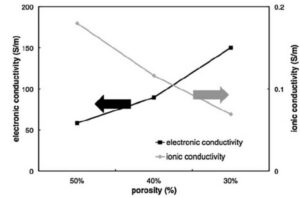
Kiʻi 2 Schematic diagram o ka porosity a me ka lithium ion a me ka conductivity electron
2. Ke ʻano a me ka ʻike ʻana i nā hemahema o ka pou
I kēia manawa, i ke kaʻina o ka hoʻomākaukau ʻana i nā pou pākaukau, ʻoi aku ka nui o nā ʻenehana ʻike pūnaewele, i mea e ʻike pono ai i nā hemahema o ka hana ʻana o nā huahana, hoʻopau i nā huahana kīnā ʻole, a me nā manaʻo kūpono i ka laina hana, hoʻoponopono ʻokoʻa a manual paha i ka hana. kaʻina hana, e hoʻemi i ka helu hemahema.
ʻO nā ʻenehana ʻike ma ka laina i hoʻohana mau ʻia i ka hana ʻana i ka pole sheet, ʻo ia ka slurry character detection, pole sheet quality detection, dimension detection a pēlā aku, No ka laʻana: (1) ua hoʻokomo pololei ʻia ka mika viscosity pūnaewele i loko o ka pahu mālama uhi e ʻike ai i ka rheological. nā hiʻohiʻona o ka slurry i ka manawa maoli, E ho'āʻo i ka paʻa o ka slurry; (2) Ke hoʻohana nei i ka X-ray a i ʻole β -ray i ke kaʻina o ka uhi ʻana, ʻO kona pololei ana kiʻekiʻe, akā nui ka radiation, ke kumukūʻai kiʻekiʻe o nā lako a me nā pilikia mālama; (3) Hoʻohana ʻia ka ʻenehana ana ʻana i ka mānoanoa o ka ʻenehana Laser online no ke ana ʻana i ka mānoanoa o ka pepa pou, Hiki ke ana i ka pololei o ± 1. 0 μm, hiki iā ia ke hōʻike i ke ʻano hoʻololi o ke ana ʻana o ka mānoanoa a me ka mānoanoa i ka manawa maoli. a me ka nānā 'ana; (4) ʻenehana hiʻohiʻona CCD, ʻo ia hoʻi, hoʻohana ʻia ka laina laina CCD e nānā i ka mea i ana ʻia, ʻO ka hoʻoili kiʻi manawa maoli a me ka nānā ʻana i nā ʻāpana kīnā, E ʻike i ka ʻike pūnaewele ʻole o ka pole sheet surface defects.
Ma ke ʻano he mea hana no ka mālama ʻana i ka maikaʻi, pono ka ʻenehana hoʻāʻo pūnaewele e hoʻomaopopo i ka pilina ma waena o nā hemahema a me ka hana pākaukau, i mea e hoʻoholo ai i nā koina kūpono / kūpono ʻole no nā huahana semi-finished.
Ma ka ʻaoʻao hope, ua hoʻolauna pōkole ʻia ke ʻano hou o ka ʻenehana ʻike kīnā ʻili o ka pā lithium-ion, ka ʻenehana infrared thermal imaging a me ka pilina ma waena o kēia mau hemahema like ʻole a me ka hana electrochemical.
(1) Nā hemahema maʻamau ma luna o ka ʻili pepa kia
Hōʻike ka Figure 3 i nā hemahema maʻamau ma ka ʻili o ka lithium ion battery electrode, me ke kiʻi optical ma ka hema a me ke kiʻi i hopu ʻia e ke kiʻi thermal ma ka ʻākau.
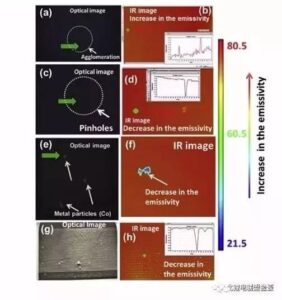
Kiʻi 3 Nā hemahema maʻamau ma luna o ka ʻili o ka pepa kia: (a, b) pōpō puʻupuʻu / aggregate; (c, d) hoʻokuʻu mea / ʻohu; (e, f) metala haole kino; (g, h) ka uhi like ole
(A, b) hoʻokiʻekiʻe ʻia ka bulge / aggregate, hiki ke loaʻa ia mau hemahema inā hoʻoulu ʻia ka slurry a ʻaʻole paʻa ka wikiwiki o ka uhi. ʻO ka hui ʻana o nā mea hoʻopili a me ka carbon black conductive agents e alakaʻi i ka haʻahaʻa haʻahaʻa o nā mea hana a me ke kaumaha māmā o nā papa polar.
(c, d) hāʻule / pinhole, ʻaʻole i uhi ʻia kēia mau wahi hemahema a hana pinepine ʻia e nā ʻōhū i loko o ka slurry. Hoʻemi lākou i ka nui o nā mea hana a hōʻike i ka mea hōʻiliʻili i ka electrolyte, pēlā e hōʻemi ai i ka mana electrochemical.
(E, f) nā kino haole metala, slurry a me nā mea ʻē aʻe i hoʻokomo ʻia i loko o ka mea hana a me ke kaiapuni, a hiki i nā kino haole metala ke hōʻeha nui i nā pā lithium. Hoʻopilikia pololei nā ʻāpana metala nui i ka diaphragm, ka hopena i kahi kaapuni pōkole ma waena o nā electrodes maikaʻi a maikaʻi ʻole, ʻo ia ke kaapuni pōkole kino. Eia kekahi, ke hui pū ʻia ke kino haole metala i ka electrode maikaʻi, piʻi ka hopena maikaʻi ma hope o ka hoʻopiʻi ʻana, hoʻonā ka metala, hoʻolaha i ka electrolyte, a laila e hoʻoheheʻe ʻia ma ka ʻaoʻao maikaʻi ʻole, a hope loa e puncture i ka diaphragm, hana i kahi pōkole pōkole. ʻo ia ka pōkole hoʻoheheʻe kemika. ʻO nā kino ʻē aʻe metala maʻamau i ka pūnaewele hale hana pākaukau ʻo Fe, Cu, Zn, Al, Sn, SUS, etc.
(g, h) ka uhi ʻole ʻia, e like me ka hui ʻana o ka slurry ʻaʻole lawa, maʻalahi ka ʻike ʻana i ka ʻili o ka ʻāpana i ka nui o ka ʻāpana, ka hopena i ka uhi ʻole ʻia, e hoʻopilikia i ka kūlike o ka mana o ka pākaukau, a ʻike maoli ʻia. ʻaʻohe kāʻei uhi, he hopena i ka hiki a me ka palekana.
(2) Hoʻohana ʻia ka ʻenehana kiʻi wela infrared (IR) no ka ʻike ʻana i nā hemahema liʻiliʻi ma nā electrodes maloʻo e hiki ke hōʻino i ka hana ʻana o nā pā lithium-ion. I ka wā o ka ʻike pūnaewele, inā ʻike ʻia ka defect electrode a i ʻole pollutant, e kaha iā ia ma ka pepa pole, e hoʻopau iā ia i ke kaʻina hana aʻe, a e hoʻopuka i ka laina hana, a hoʻoponopono i ke kaʻina hana i ka manawa e hoʻopau ai i nā hemahema. ʻO ka hihi infrared he ʻano hawewe electromagnetic i like ke ʻano me nā hawewe lekiō a me ke kukui ʻike ʻia. Hoʻohana ʻia kahi mea uila kūikawā e hoʻololi i ka mahele wela o ka ʻili o kahi mea i ke kiʻi ʻike ʻia o ka maka o ke kanaka, a no ka hōʻike ʻana i ka puʻunaue wela o ka ʻili o kahi mea i nā kala like ʻole i kapa ʻia ʻo ka ʻenehana kiʻi wela infrared. Kapa ʻia kēia mea uila uila infrared thermal imager. ʻO nā mea a pau ma luna o ka ʻeleʻele loa (-273 ℃) e hoʻokuʻu i ka radiation infrared.
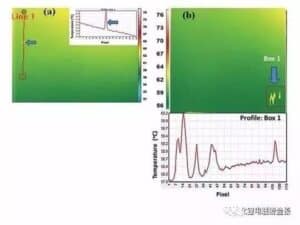
E like me ka mea i hōʻike ʻia ma ka Figure 4, hoʻohana ka infrared thermal approximator (IR Camera) i ka mea ʻike infrared a me ka pahuhopu kiʻi optical e ʻae i ke ʻano hoʻohele ikehu infrared radiation o ka mea i ana ʻia a noʻonoʻo iā ia ma ka mea photosensitive o ka mea ʻike infrared e loaʻa ai ka kiʻi wela infrared, e pili ana i ke kahua mahele wela ma ka ʻili o ka mea. Ke loaʻa kahi kīnā ma ka ʻili o kahi mea, hoʻololi ka mahana ma ia wahi. No laila, hiki ke hoʻohana ʻia kēia ʻenehana no ka ʻike ʻana i nā hemahema ma ka ʻili o ka mea, kūpono loa no kekahi mau hemahema ʻaʻole hiki ke hoʻokaʻawale ʻia e nā ala ʻike ʻike. Ke ʻike ʻia ka electrode maloʻo o ka pākaukau lithium ion ma ka pūnaewele, e hoʻomālamalama mua ʻia ka electrode electrode e ka uila, hoʻololi ka mahana o ka ʻili, a laila ʻike ʻia ka mahana o ka ʻili me kahi kiʻi thermal. ʻIke ʻia ke kiʻi puʻunaue wela, a hoʻoponopono ʻia ke kiʻi a hoʻopaʻa ʻia i ka manawa maoli e ʻike ai i nā hemahema o ka ʻili a hōʻailona iā lākou i ka manawa.D. Mohanty Ua hoʻokomo ka haʻawina i kahi kiʻi kiʻi wela ma ka puka o ka umu hoʻomaloʻo coater e ʻike i ke kiʻi puʻunaue wela o ka ʻili pepa electrode.
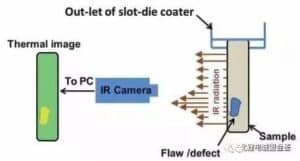
He kiʻi 5 (a) he palapala ʻāina hoʻohele wela o ka ʻili i uhi ʻia o ka pepa kia maikaʻi NMC i ʻike ʻia e ka mea kiʻi wela, aia kahi kīnā liʻiliʻi ʻaʻole hiki ke ʻike ʻia e ka maka ʻōlohelohe. Hōʻike ʻia ka puʻupuʻu puʻupuʻu wela e pili ana i ka ʻāpana ala i loko o ka inset i loko, me ka piʻi ʻana o ka wela ma ka wahi hemahema. Ma ke Kiʻi 5 (b), piʻi ka wela ma ka ʻāina i loko o ka pahu e pili ana, e pili ana i ka hemahema o ka ʻili pole. FIG. ʻO 6 he kiʻi mahele wela o ka ʻili o ka pepa electrode maikaʻi ʻole e hōʻike ana i ke ola ʻana o nā hemahema, kahi o ka piʻi ʻana o ka wela e pili ana i ka ʻōhū a i ʻole ka hōʻuluʻulu ʻana, a ʻo ke ʻano o ka emi ʻana o ka mahana e like me ka pinhole a i ʻole hāʻule.
Kiʻi 5 Ka mahele wela o ka ʻili pepa electrode maikaʻi
Kiʻi 6 Ka mahele wela o ka ʻili electrode maikaʻi ʻole
Hiki ke ʻike ʻia ʻo ka ʻike kiʻi wela o ka hāʻawi ʻana i ka mahana he ala maikaʻi ia o ka pole sheet surface defect detection, hiki ke hoʻohana ʻia no ka hoʻomalu maikaʻi ʻana i ka hana ʻana o ka pole sheet.3. ʻO ka hopena o nā hemahema o ka ʻili o ka pole ma ka hana ʻana o ka pākaukau
(1) Ka hopena i ka mana hoʻonui pākahi a me ka pono Coulomb
Hōʻike ka Kiʻi 7 i ka pihi mana o ka aggregate a me ka pinhole ma ka mana hoʻonui pākaukau a me ka pono o ka coulen. Hiki i ka aggregate ke hoʻomaikaʻi maoli i ka mana pākaukau, akā e hōʻemi i ka pono coulen. ʻO ka pinhole e hoʻemi ana i ka mana pākaukau a me ka pono Kulun, a ua emi nui ka maikaʻi Kulun ma ke kiʻekiʻe.
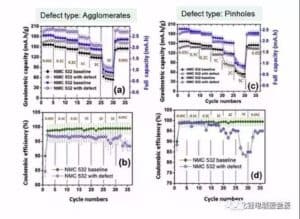
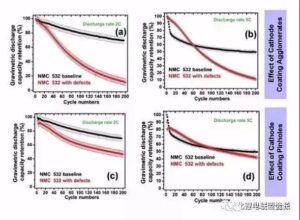
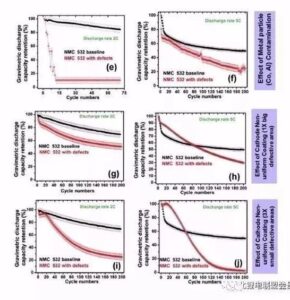
Figure 7 cathode aggregate a me ka hopena pinhole ma ka mana pākaukau a me ka pono o ka helu 8 he uneven coating, a me ka metala haole kino Co a me Al ma luna o ka pākaukau kaha a me ka hopena o ka pono curve, uneven coating e hoemi i ka nuipa o ka 10% - ʻO 20%, akā ua emi ka nui o ka pākaukau holoʻokoʻa e 60%, hōʻike kēia i ka emi ʻana o ka nui o ke ola ma ka ʻāpana polar. Metal Co haole kino i hoemi 'ia ka mana a me Coulomb pono, a hiki i ka 2C a me 5C kiʻekiʻe magnification,ʻaʻohe hiki i nā mea a pau, i hiki ma muli o ke kukuluia'na o metala Co i ka electrochemical hopena o ka lithium a me ka lithium i hoʻokomoʻia, a i ole ia paha na metala particles. Ua ālai ʻia ka puka diaphragm ma muli o ke kaapuni pōkole micro.
Kiʻi 8 Nā hopena o ka uhi ʻole ʻana o ka electrode maikaʻi a me nā kino ʻē aʻe Co a me Al ma luna o ka mana hoʻonui pākahi a me ka pono coulen.
ʻO ka hōʻuluʻulu manaʻo o nā hemahema pepa cathode: ʻO nā ʻai i loko o ka uhi uhi cathode e hōʻemi i ka pono Coulomb o ka pākaukau. ʻO ka pinhole o ka uhi maikaʻi e hōʻemi i ka maikaʻi o Coulomb, ka hopena i ka hana hoʻonui maikaʻi ʻole, ʻoi aku ka nui o ka nui o kēia manawa. Ua hōʻike maikaʻi ʻole ka hoʻopili heterogeneous i ka hana hoʻonui. Hiki i nā mea hoʻohaumia metala ke hoʻoulu i nā pōkā pōkole micro, a no laila hiki ke hoʻemi nui i ka hiki o ka pākaukau.
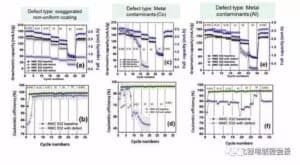
Hōʻike ka Kiʻi 9 i ka hopena o ka leakage foil strip maikaʻi ʻole i ka mana multiplier a me ka pono Kulun o ka pākaukau. Ke hiki mai ka leakage ma ka electrode maikaʻi ʻole, ua hoʻemi nui ʻia ka mana o ka pākaukau, akā ʻaʻole maopopo ka mana gram, a ʻaʻole nui ka hopena i ka pono Kulun.
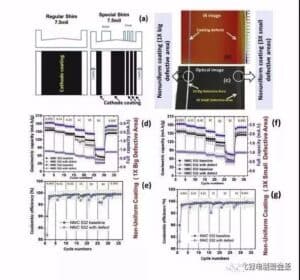
Kiʻi 9 Ka hoʻoikaika ʻana o ka ʻōpala leakage electrode maikaʻi ʻole i ka mana hoʻonui pākahi a me ka maikaʻi o Kulun (2) ʻO ka hoʻoikaika ʻana i ka hana pōʻaiapili hoʻonui pākaukau ʻO ke kiʻi 10 ka hopena o ka hopena o ka pōʻino o ka ʻili uila ma ka pōʻai hoʻonui pākaukau. Ua hōʻuluʻulu ʻia nā hopena hopena penei:
Egregation: ma 2C, ʻo 200% ka nui o ka mālama ʻana o 70 cycles a ʻo 12% ka pākaukau kīnā ʻole, ʻoiai i ka pōʻai 5C, ʻo ka nui o ka mālama ʻana o 200 cycles he 50% a ʻo ka pākaukau hemahema he 14%.
Needlehole: ʻike ʻia ka hiki ke attenuation, akā ʻaʻole wikiwiki ka attenuation defect aggregate, a ʻo ka nui o ka mālama ʻana o 200 cycles 2C a me 5C he 47% a me 40%, kēlā me kēia.
ʻO ke kino haole metala: ʻaneʻane 0 ka nui o ka metala Co kino haole ma hope o kekahi mau pōʻaiapuni, a ʻo ka 5C pōʻai o ke kino haole metala Al foil e emi nui ana.
Leak Strip: No ka wahi leaka hoʻokahi, ʻoi aku ka wikiwiki o ka nui o ka pākaukau o nā kaha liʻiliʻi liʻiliʻi ma mua o kahi kaha ʻoi aku ka nui (47% no 200 mau pōʻai ma 5C) (7% no 200 pōʻai i 5C). Hōʻike kēia i ka nui o ka nui o nā paʻi, ʻoi aku ka nui o ka hopena i ka pōʻai pālolo.
Kiʻi 10 Ka hopena o nā hemahema o ka ʻili uila ma ka pōʻaiapili helu kelepona
Ref.: [1] ʻO ka loiloi hoʻopōʻino ʻole o ka slot-die-coated lithium secondary batteryelectrodes e in-line laser caliper a me IR thermography method [J].ANALYTICALMETHODS.2014, 6(3): 674-683.[2]Effect o electrode manufacturing defects on electrochemical performance of lithium-ion batteries: Cognizance of the battery failure sources [J]. Journal of Power Sources.2016, 312: 70-79.



